|
Water in Nature
>> All three phases of water exist
naturally in the atmosphere
Hydrologic Cycle: Closed Atmospheric System
>> Evaporation requires energy to
change liquid to gas: 597 calories/gram
>> Condensation releases energy when gas turns to liquid: 597 calories/gram
>> Melting requires energy to change solid to liquid: 80 calories/gram
>> Freezing releases energy when liquid turns to solid: 80 calories/gram
>> Sublimation requires energy to change solid to gas: 677 calories/gram
(597+80)
>> Deposition releases energy when gas turns to solid: 677 calories/gram
(597+80)
A diagram of the various phase changes
and the energy needed to complete these changes can be found below in Figure
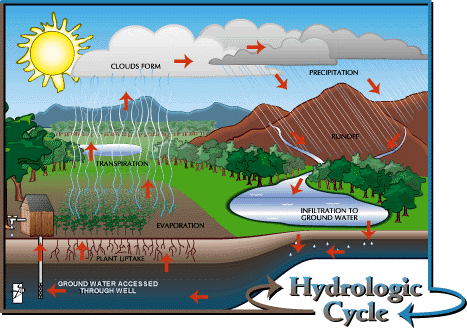
1. Figure 2 depicts the earth's hydrologic cycle.
|
|
|
Figure 1:
Energy diagram for the phase changes of water. Energy is required for upward-moving phase changes, energy is released
for downward-moving processes. |
|
 |
|
Figure 2:
The image above illustrates how the phase changes outlined in Figure 1 are
all interconnected in the hydrologic cycle. |
Humidity: Amount of Water Vapor in the Air
>> Relative Humidity = (Vapor
Pressure/Saturation Vapor Pressure) * 100
The relative humidity is a measure of
the amount of water vapor in the air compared to (divided by) the amount of water vapor in the air if it were saturated.
>> Saturation occurs when the evaporation rate equals the rate of condensation.
|
|
|
Figure 3:
The application above demonstrates the relationship between the rates of
evaporation and condensation and the saturation vapor pressure. |
Once the air reaches the saturation
point, the rate of evaporation becomes equal to the rate of condensation.
Also, it is at this point when the relative humidity equals 100 percent and the
temperature and dewpoint are identical. The concept of air saturation and
the saturation vapor pressure can be further explained by the demonstration
found to the right in Figure 3.
Note: The terms Saturation Vapor Pressure and Equilibrium Vapor Pressure are interchangeable
>> Saturation vapor pressure increases
as temperature increases
 |
|
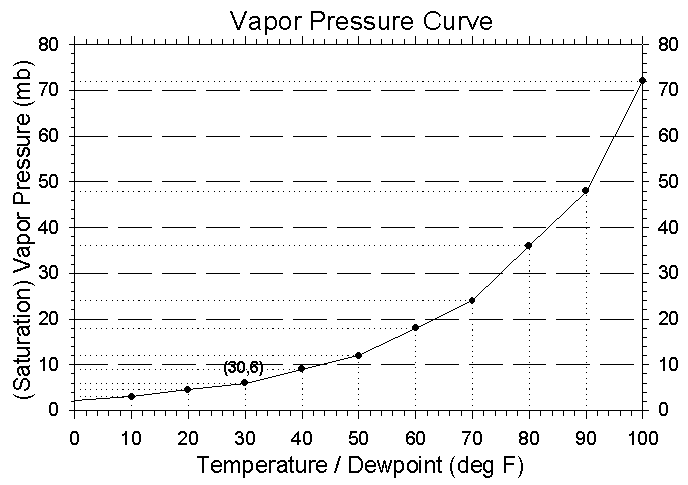
Figure 4:
The graph found above plots the saturation vapor pressure versus the
temperature in degrees Fahrenheit. |
The saturation vapor pressure changes as the temperature changes - approximately
doubling for every 20 degree F increase in temperature. The approximate
value of the saturation vapor pressure at 30 degrees Fahrenheit is 6 millibars.
This approximation can be found in the graph to the left.
>> Relationship between temperature,
dewpoint and relative humidity
The temperature, dewpoint and relative
humidity values are all connected to one another through vapor pressures.
In order to calculate the relative humidity, the actual vapor pressure and the
saturation vapor pressure must be calculated. Through the use of
the graph above, the dewpoint and temperature can be used to determine the actual and
saturation vapor pressures respectively. Armed with this information, the
relative humidity can be calculated using the equation found above.
Cloud Formation
>> Cool the air to the saturation
point (dewpoint)
Cloud formation is dependent upon the
cooling of air to the saturation point. Once net condensation occurs, a cloud will form.
This cooling can be accomplished two ways - either through radiational cooling, or through
rising air. The formation of clouds through radiational cooling is
most common right near the surface of the earth. When clouds form at the surface,
the result is known as fog.
The other method of cloud formation,
forcing air to rise and cool, can be accomplished in several different ways.
The rising bubbles of warm, buoyant air in convection will produce clouds if sufficient water
vapor is present. The convergence of winds into the center of a low pressure system, and the process of
orographic lifting will also produce clouds if the air is sufficiently moist. In the case of
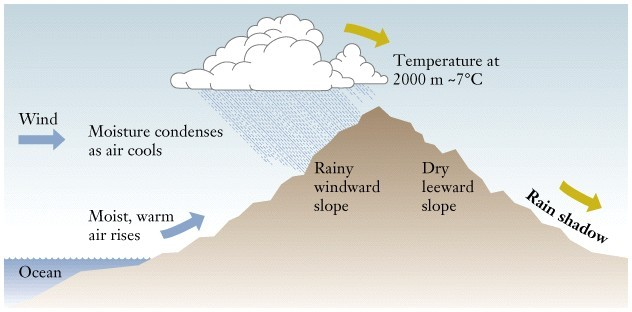
orographic lifting, a change (increase) in elevation of the earth's surface forces air to
rise and consequently cool. This may eventually lead to heavy precipitation on the
windward side of a mountain and a rain shadow on the leeward side.
Examples of convection and orographic lifting can be found below.
|
 |
 |
|
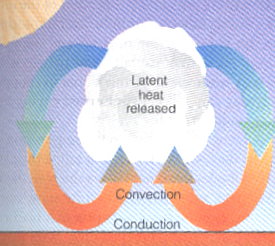
Figure 5:
The image above shows the process of convection, where warm bubbles
of air rise. Eventually, if moisture is plentiful, condensation will produce clouds as the air cools to the dewpoint. |
Figure 6:
This image illustrates orographic lifting and the resultant formation of clouds and precipitation on the windward side of the mountain. |
>> Condensation nuclei (seeds)
Some of the most important
ingredients needed to form a cloud are cloud condensation nuclei.
These tiny particles of dust, dirt, salt and other assorted materials act as the "seeds"
of a cloud by providing a surface on which water vapor from the air can condense.
Precipitation Formation
>> The Warm Rain Process: Collision and
Coalescense
|
 |
|
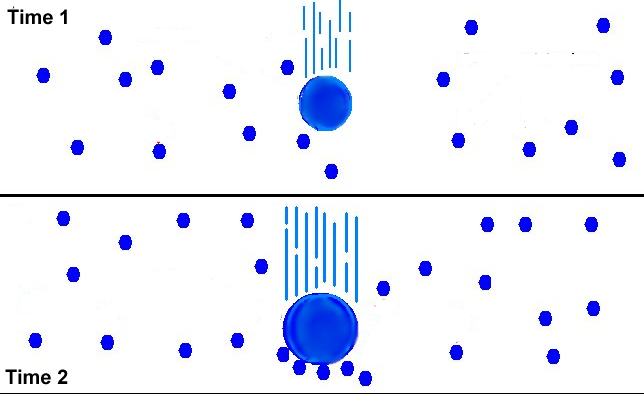
Figure 7:
This figure illustrates the collision and coalescense process of raindrop
formation. As air currents force tiny water droplets to collide, some grow
large enough to begin to fall. As they fall, they collide with and absorb
smaller drops - eventually falling out of the cloud as drops of rain. |
The formation of precipitation through collision and coalescense is a process
reserved for only clouds containing no ice crystals. Consequently, only
the tropics routinely receive much of their precipitation through collision and coalescense.
This formation process relies on the fact that the larger water drops
will fall faster than the smaller water drops. This will cause the larger
drops to collide with and absorb the smaller drops until eventually a
raindrop is formed. This process can be seen in Figure 7 to the right.
>> Bergeron-Findeisen Process: All 3
phases of water can (and do) exist in cold clouds
In colder climates, all three phases
of water exist in a cloud at one time and, therefore, the process of collision
and coalescense is overshadowed by one that is more efficient- the Bergeron-Findeisen Process.
At the beginning of this more complex precipitation
formation process, there is much less ice in a cloud than liquid water. This is because ice
nuclei in the atmosphere are sparse. This imbalance will change as time goes on,
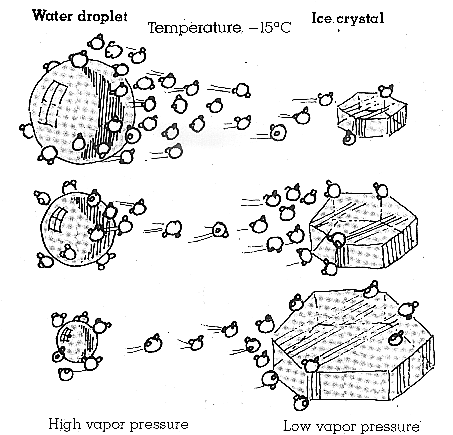
however, because the vapor pressure over ice is less than that over
water at the same temperature. This property, which can be seen in
Figure 8, below. causes the ice crystals to grow at the expense of the water
droplets. Eventually, the ice crystals become large enough that they begin
to fall. As these crystals fall, one of two things can happen - they
either grow larger from colliding with water droplets, or they collide with
other ice crystals and shatter to form new ice nuclei. If temperatures are
high enough the ice crystals that fall through the cloud melt and eventually fall out as rain.
|
 |
|
Figure 8:
The image above illustrates the growth of an ice crystal at the expense of a
water droplet through the Bergeron-Findeisen Process of precipitation
formation. |
|